We daily come across high pressures exerted on small surfaces. Let us estimate, for example, what the pressure will be at the point of a needle. Assume that the tip of a needle or nail has a linear dimension of 0.1 mm. This implies that the area of the point will be about 0.0001 cm\(^3\). If a rather modest force of 10 kgf acts on such a nail, then the tip of the nail will exert a pressure of 100,000 atm. It’s no wonder that the pointed objects so easily penetrate deeply into dense bodies.
It follows from this example that to create high pressures on small surfaces is quite a common thing. The situation is completely different if the question is to create high pressures on large surfaces.
The creation of high pressures under laboratory conditions is accomplished with the aid of powerful presses, for example, hydraulic ones Figure 1. The force of the press is transmitted to a piston of small area, and the piston forces its way into the vessel within which we wish to create a high pressure.
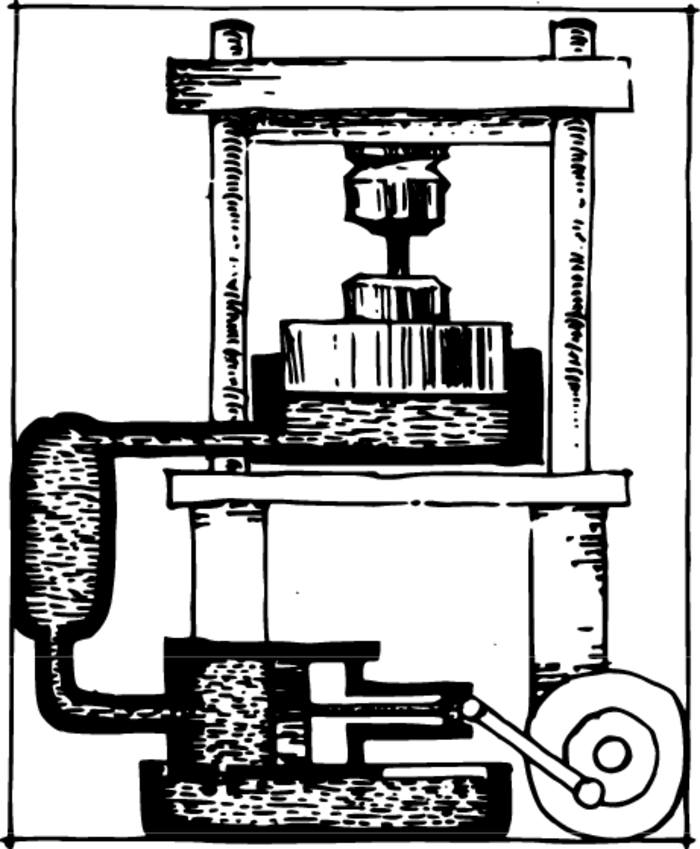
Pressures of several thousand atmospheres can be created in this manner without any particular difficulty. But in order to obtain ultrahigh pressures, we must complicate the experiment, since the material composing the vessel cannot withstand such pressures.
Here nature has met us half-way. It turns out that metals become considerably stronger under pressures of the order of 20,000 atm. Therefore, an apparatus for obtaining ultrahigh pressures is submerged in a liquid which is under a pressure of the order of 30,000 atm. In this case, one is able to create pressures of several hundred thousands of atmospheres (but again with a piston). The highest pressure—400,000 atm—was obtained by the American physicist Percy Williams Bridgman.1
Our interest in obtaining ultrahigh pressures is far from idle. Phenomena which are impossible to induce by other methods can occur at such pressures. Artificial diamonds were obtained in 1955. A pressure of 100,000 atm and, in addition, a temperature of 2000 K were required for this. Ultrahigh pressures of the order of 300,000 atm on large surfaces are formed during explosions of solid or liquid explosive materials—nitroglycerine, trotyl, etc. Incomparably higher pressures attaining \(10^{13}\) atm arise, within an atomic bomb during its explosion. Pressures during an explosion exist for a very short time.
There are constant high pressures deep inside celestial bodies including the Earth, of course. The pressure at the centre of the Earth is equal to approximately 3 million atmospheres.
- This value is based on older standards and may not reflect advancements in modern vacuum technology.↩︎