We live on the bottom of an ocean of air—the atmosphere. Each body, every grain of sand, any object situated on the Earth is subject to air pressure.
Atmospheric pressure isn’t so small. A force of about kgf acts on each square centimetre of a body’s surface. The cause of atmospheric pressure is obvious. Just as water, air possesses weight and, therefore, exerts a pressure equal (just as for water) to the weight of the column of air above the body. The higher we climb up a mountain, the less air there will be above us and, therefore, the lower will atmospheric pressure become. One must know how to measure pressure for scientific and everyday purposes. There exist special instruments—barometers— for this.
It isn’t difficult to make a barometer. Mercury is poured into a tube with one end sealed off. Closing the open end with a finger, one turns the tube upside-down and submerges its open end in a cup of mercury. When this is done, the mercury in the tube will fall, but will not all pour out. The space above the mercury in the tube is undoubtedly airless. The mercury is supported in the tube by the pressure of the external air Figure 1.
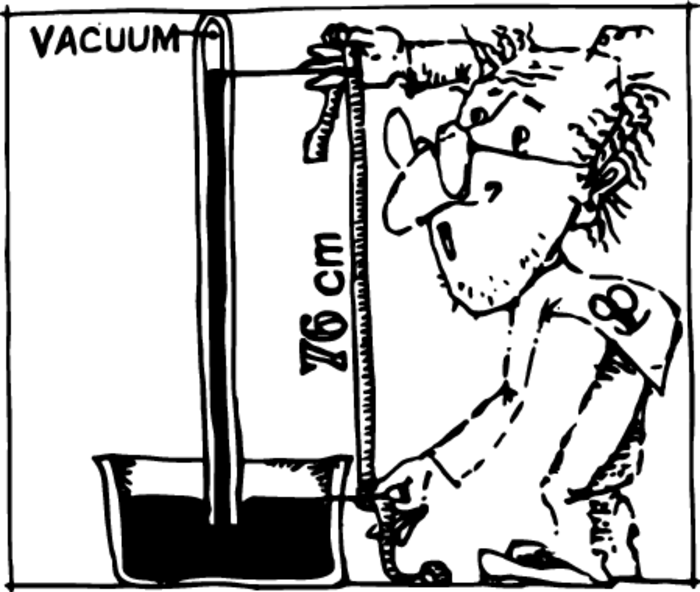
Whatever the dimensions of the cup with mercury, and whatever the diameter of the tube, the mercury will stand at about one and the same height—76 cm.
If we take a tube shorter than 76 cm, it will be completely filled by mercury and we will not see any vacuum. A 76 cm column of mercury presses down on the support with the same force as the atmosphere. This mercury column with a cross-sectional area of 1 cm\(^2\) presses down with a force of 1.033 kgf. This number is the volume of the mercury \(1 \times 76~\mathrm{m^3}\) multiplied by its density and the acceleration of free fall.
As you see, the average atmospheric pressure (usually called standard atmospheric pressure) that is exerted on everything on the Earth is close to the pressure that 1 kg weight exerts on an area of 1 cm\(^2\).
Various units are used in measuring pressures. One often simply indicates the height of a mercury column in millimetres. For example, we say that the pressure is above normal today, it is equal to 768 mm Hg (i.e. of mercury).
A pressure of 760 mm Hg is sometimes called a standard atmosphere. A pressure of 1 kgf/cm\(^2\) is called a technical atmosphere. Since the difference between a physical atmosphere and a technical atmosphere is very small, from now on we will not distinguish between them.
Physicists also make frequent use of another unit of pressure, the bar; \[1~\mathrm{bar} =10^6~\mathrm{dyn/cm^2}\] Since 1 gf = 981 dyn, one bar is approximately equal to one atmosphere. More precisely, standard (normal) atmospheric pressure roughly equals 1013 millibars.
The unit of pressure in the SI system is the pascal (Pa), which is the pressure produced by a force of 1 N acting on an area of 1 m\(^2\). This is very little pressure, as can be seen from the fact that \[1~\mathrm{Pa} = 1~\mathrm{N/m^2} = 10\,\,\mathrm{dyn/cm^2} = 10^{-5}\,\, \textrm{bar}.\] Computing the area of the Earth’s surface with the aid of the formula \(4 \pi R^{2}\) , we find that the weight of the entire atmosphere is expressed by the enormous figure of \(5\times 10^{18}\) kgf.
Barometer tubes can have the most varied forms; only one thing is important: one of the ends of the tube must be sealed off in such a way that there be no air above the surface of the mercury. Atmospheric pressure acts on the other level of the mercury.
Atmospheric pressure can be measured by a mercury barometer with a very great accuracy. Of course, it isn’t necessary to use only mercury; any other liquid is suitable. But mercury is the heaviest liquid, and so the height of a mercury column under standard pressure will be minimum. The mercury barometer is not a particularly convenient instrument. It is not good to leave a surface of mercury open (mercury vapour is poisonous); further- more, this instrument is not portable.
These drawbacks are not shared by aneroid barometers—aneroids (i.e. airless). Everyone has seen such a barometer. It is a small round metal box with a scale and a pointer. Values of pressure are marked on the scale, usually in centimetres of a mercury column. The air has been pumped out of the metal box. The cover of the box is kept in place by a strong spring, since it would otherwise be crushed by atmospheric pressure.
With a change in atmospheric pressure, the cover either bends or straightens. The pointer is connected to the cover in such a manner that the pointer moves to the right when the cover is bent.
Such a barometer is graduated by comparing its readings with those of a mercury barometer. If you want to know the pressure, don’t forget to knock on the barometer with your finger. The pointer of the dial experiences considerable friction and usually gets stuck at “yesterday’s weather”.
A simple mechanism—the siphon—is based on atmospheric pressure,
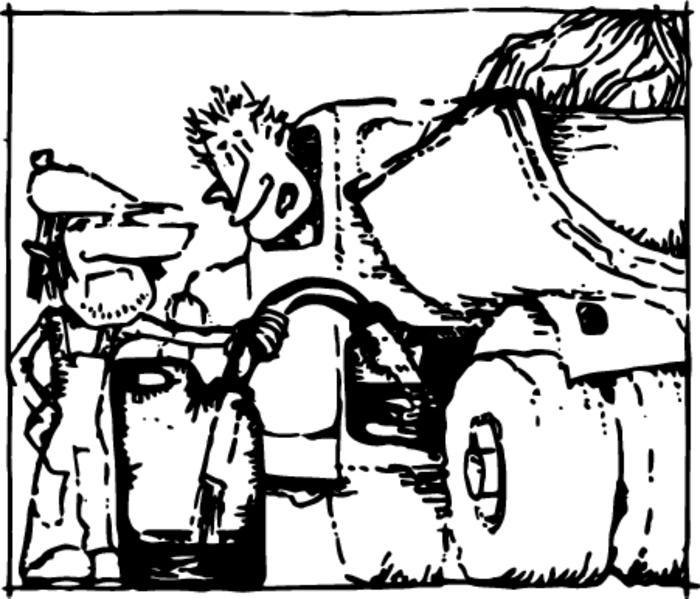
A driver wants to help his friend, who is out of gas. But how can gasoline be poured from the tank of his car? It can’t be inclined like a tea-kettle.
A rubber tube comes to his aid. He lowers one of its ends into his gas tank and orally sucks the air out of the other end. Then a rapid motion-the open end is stopped up with a finger and placed at a height below the gas tank. Now the finger can be removed—the gasoline will pour out of the hose Figure 2.
A bent rubber tube is just what a siphon is. The liquid moves in this case for the same reason as through a straight inclined tube. In the final analysis, the liquid flows downwards in both cases.
Atmospheric pressure is necessary for the action of a siphon: it “props up” the liquid and doesn’t let the column of liquid in the tube break. If there were no atmospheric pressure, the column would break at the transfer point and the liquid would slip into both vessels.
The siphon starts functioning when the liquid in the right-hand (i.e. the “pouring”) part of the tube drops below the level of the liquid being siphoned off, into which the left end of the tube has been lowered. The liquid would otherwise flow back.